Delta resurgence reignites COVID-19 research
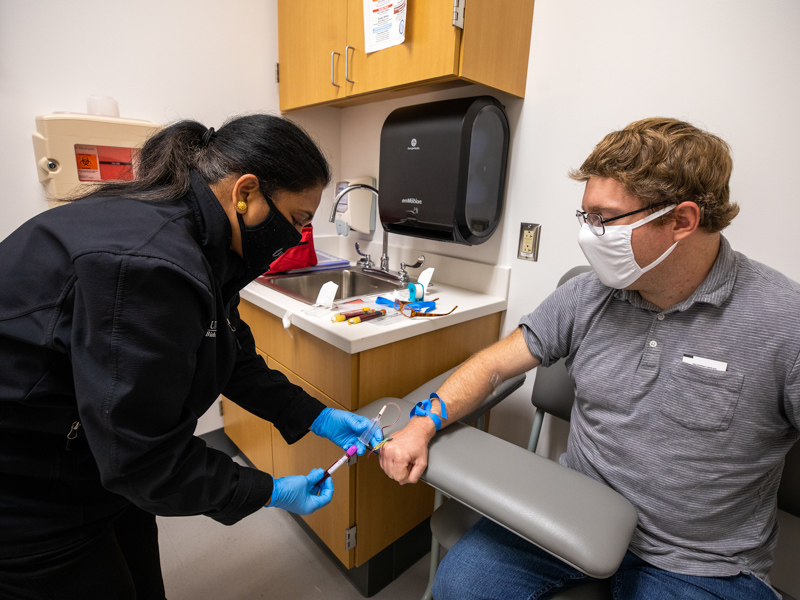
For a short time in spring 2021, researchers who took up the mantle of COVID-19 prepared for a return to their regular expertise.
With three authorized vaccines, multiple treatment strategies and daily new case counts down 90 percent from their January peaks, some pandemic research at University of Mississippi Medical Center started to reach the end of its life cycle.

“At this time last year, we had about 20 clinical trials related to COVID-19 happening at the Medical Center. After the winter surge, we knew some of our trials were going to end,” said Dr. Gailen Marshall, R. Faser Triplett, Sr. M.D. Chair of Allergy and Immunology.
Some of that was due to decreased cases and hospitalizations limiting recruitment opportunities. Other studies reached their predetermined endpoints or stopped because the treatments they tested were not effective.
Then came Delta.

“This new variant has put COVID-19 research back into the spotlight,” said. Dr. James Galbraith, associate professor of emergency medicine.
UMMC Emergency Medicine has been heavily involved in COVID-19 research. The department enrolled the Medical Center’s first clinical trial participant in March 2020. Today, more than 800 people have been part of the department’s pandemic-associated studies covering “therapeutics, diagnostics, registries and epidemiology,” said Galbraith, research director for the department.
Today, UMMC’s clinical trials database lists 17 COVID-19 studies recruiting participants or preparing to activate, many of which are tied to emergency and critical care. Galbraith says there are more in the pipeline.

Dr. Utsav Nandi, assistant professor of emergency medicine and associate research director for the department, said UMMC participates in multiple studies as part of the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) network. One covers the use of antithrombotic drugs, which limit blood clotting, as a way to limit the risk of blood clotting in hospitalized COVID-19 patients. Another project focuses on monoclonal antibodies.
UMMC’s ED is also part of the Preventing Emerging Infections through Vaccine EffectiveNess Testing-COVID (PREVENT) trial, which is studying UMMC health care workers to understand how well each of the authorized vaccines work.
Clinical trials are an important tool in finding effective prevention and treatment methods for COVID-19. However, they are hardly the only pandemic research projects happening at the Medical Center.

The Molecular and Genomics Core Facility uses advanced sequencing technology to track SARS-CoV-2 variants. Its surveillance helped document the rise of Delta during early summer 2021. Through the next year, the team will continue to monitor the emergence of other variants both in real-time and through a retrospective study of the pandemic.
In July, UMMC scientists collaborated with the Massachusetts Institute of Technology and Harvard University to publish a paper using cells from diagnostic nasal swabs collected in 2020 to predict the course of COVID-19 disease. Dr. Sarah Glover, professor of medicine and senior co-author of the paper, said the team is doing a follow-up study to compare these initial findings to patients with Delta-variant COVID-19 or breakthrough infections post-vaccination.
Dr. Ritesh Tandon, associate professor of microbiology and immunology, is collaborating with University of Mississippi scientists to study the use of a heparin-based nasal spray to prevent SARS-CoV-2 infections from taking hold.

The team has studied the safety and pharmacokinetics of the sugars like heparin, which can bind to the virus in ways that limit its ability to enter cells and replicate. They were able to conduct a pre-clinical trial using this method. However, “A few months ago, there was some waning in interest in this research and we did not have enough patients to conduct clinical trials,” Tandon said.
Now COVID-19 has come back with a vengeance, and we need antiviral treatments in addition to vaccines. The NIH is putting more emphasis on funding for antiviral research on COVID-19,” he said, adding that he and his collaborators are seeking federal funding to continue the work.
In addition, “We are also starting to see [private industry] sponsors who we worked with previously come back with interest in doing more trials with us,” said Marshall, the medical director for UMMC’s Clinical Research and Trials Unit.
Marshall’s scientific work during the pandemic includes studies on the use of convalescent plasma as a treatment for COVID-19 and the potential for novel anti-inflammatory products. He and his collaborators are also continuing work on a biosurveillance program of COVID-19 antibody prevalence among UMMC personnel.
“One thing on a lot of minds right now is booster shots. Are these going to be important? If they are, for who and when should we give them? Our work is going to contribute to the body of knowledge that answers those questions,” Marshall said.
Marshall also says a group of UMMC researchers is starting to study post-acute sequelae of COVID-19, also known as “long-haul COVID.”
“About 25 to 40 percent of COVID-19 patients develop some form of long-haul COVID,” Marshall said. “Like we see in the acute phase of the disease, there is a lot of variability in what people experience. Some folks have a lingering cough or brain fog for a month or two that goes away, but others even a year later are still having symptoms.”

UMMC’s COVID-19 research efforts also go out into the community. Dr. Caroline Compretta, associate professor of preventive medicine, is the principle investigator for UMMC’s Community Engagement Alliance Against COVID-19 Disparities (CEAL). Announced in October 2020, this NIH-funded project uses existing community networks to provide trustworthy information to groups most heavily impacted by the pandemic.
Recently, CEAL distributed a survey on COVID-19 prevention behaviors, clinical trials, and social determinants of health that will help inform their work.
CEAL uses the multi-institutional network built by the Mississippi Center for Clinical and Translational Research (MCCTR) to conduct its research on barriers to vaccination, knowledge and behavior surrounding COVID-19 among African American young adults, and risk communication.

The MCCTR’s biostatistics core, including Dr. William Hillegass, assistant professor of data science, is also participating in clinical data sharing for the National COVID Cohort Collaborative. This repository aims to to create a five-year resource that will facilitate studies on the long-term health impacts of COVID-19.
UMMC is building its own repository through the COVID-19 Biobanking project. Led by UMMC Biobank research director Dr. Gouri Mahajan, this collection already has more than 6,000 specimens. The whole blood, serum, plasma and nasal swabs obtained since April 2020 come from more than 300 adult and pediatric patients who had mild to moderate COVID-19. The samples come from their hospital stays as well as return visits to UMMC’s Clinical Research and Trials Unit months, and now, a year after their diagnosis.
This kind of long-range infrastructure is going to be critical going forward because it will enable long-haul studies like the long-haul COVID-19 one Marshall described. He says that project would be a top-priority even if the pandemic were subsiding. Now it will also be happening alongside acute studies for the foreseeable future.
“COVID-19 will not be gone anytime soon. It will still be here next year,” Marshall said.
***
That brief reprieve from COVID-19 headlines – when Mississippi’s daily case counts were in the hundreds, not thousands – is due in large part to research.
UMMC has also contributed to massive jumps in pandemic response. The Medical Center was a site for the Johnson and Johnson vaccine clinical trials. Nandi also cites the value of the BinaxNOW COVID-19 self-antigen test, which the UMMC ED piloted before it received FDA approval.
“If you get one of these tests off the shelf at Walmart, that’s based on research that was carried here at UMMC,” Nandi said.
We also learn from the errors.
“The results of some clinical trials are negative, meaning we learn which treatments aren’t effective,” Nandi said. He cites UMMC’s participation in ORCHID, which showed the futility with hydroxychloroquine as a COVID-19 treatment.
“We have learned how to take better care of our patients and now have a better understanding of the nuances of COVID treatment, such as when someone needs supportive oxygen versus intubation,” Galbraith said. “We know we are doing better than we were at the beginning of the pandemic because we see our patients getting better.”


