WHO: more drugs, fewer superbugs

Published in News Stories on April 03, 2017
The World Health Organization has signaled for help in defeating a group of notorious villains.
They are the superbugs - bacteria with the ability to overcome our best defenses. The WHO published a list Feb. 27 prioritizing 12 bacterial strains in need of new antibiotics. The list is designed to encourage research and development into such drugs - the new superheroes.
The world needs these treatments: In the United States, antibiotic-resistant bacteria infect two million people and kill 23,000 each year, primarily those who are hospitalized or have weakened immunity. But these species are usually harmless, living among and within us.
Dr. Ashley Robinson, professor of microbiology and immunology at the University of Mississippi Medical Center, studies methicillin-resistant Staphylococcus aureus, or MRSA, one of WHO's “high” priority pathogens. One in five people carry benign S. aureus strains in their nose, while one in 50 people have a MRSA.
“What causes the switch from susceptible to resistant is a huge area of research,” he said.
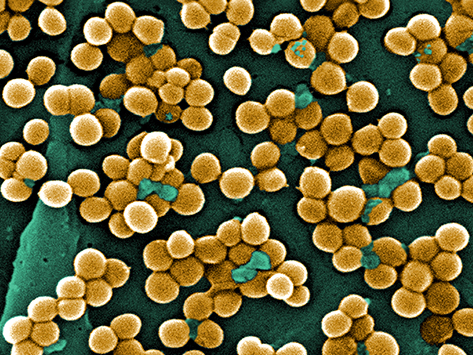
Methicillin-resistant Staphylococcus aureus, or MRSA.
Robinson studies MRSA genomics. By taking millions of DNA fragments and putting them in sequence, his lab looks for the genetic switches. His work has helped reconstruct the “family portrait” of MRSA in North America and worldwide.
“Genomics are a window into studying the transition,” he said. However, that window is more like a moving target.
“The common drug-resistant strains now are not the same ones that were common earlier,” Robinson said.
When bacteria survive antibiotic treatment, they can pass on the trait that helped them survive to their offspring. These bacteria can also survive the antibiotic attack. Because bacteria can double their population size in 30 minutes, the trait can proliferate quickly.
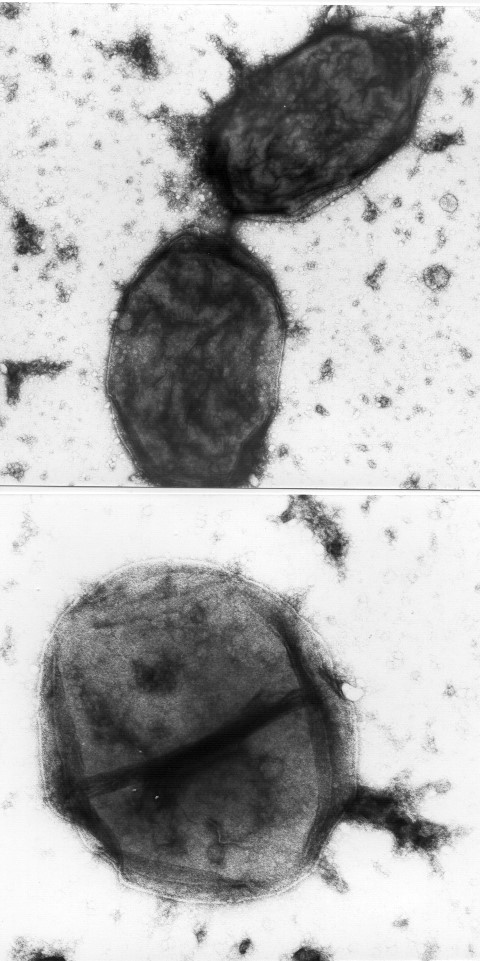
Haemophilus influenza as viewed under a microscope.
“The more we use a particular antibiotic, the less useful it becomes,” said Dr. Brian Akerley, associate professor of microbiology and immunology.
By age three, 75 percent of children get an ear infection, making this the top reason children are prescribed antibiotics. Akerley's study species, Haemophilus influenzae, is a common cause.
Akerley also uses genomic techniques to study bacteria - determining which genes can make H. influenzae antibiotic-resistant. Recently, he and colleagues have studied ways to target the bacteria using immunotherapeutics, which would bind to the bacteria and inhibit its ability to cause illness.
“The idea is to teach the pathogen to be less pathogenic,” Akerley said.
H. influenzae is listed as a “medium” priority because current treatments still work well. However, scientists have been tracking its increasing resistance to ampicillin since the 1970s.
“With every new drug, [bacteria] can figure out a way to overcome it,” said Dr. Mary Marquart, associate professor of microbiology and immunology.
Marquart studies Streptococcus pneumoniae, another WHO “medium” priority. While the extent of resistance in the species isn't “alarming” yet, she said the potential is there.
Bacteria can acquire resistance and virulence through gene transfer, she said. Cells of different species can trade DNA fragments like baseball cards. A benign denizen of your lung can become a superbug after receiving a new gene from an antibiotic-resistant neighbor.
Between antibiotic misuse and the natural abilities of bacteria, Marquart said microbiologists think we are approaching a “post-antibiotic era” - when the most resistant strains won't submit to any antibiotic. For some species, that era might be closer than we think.
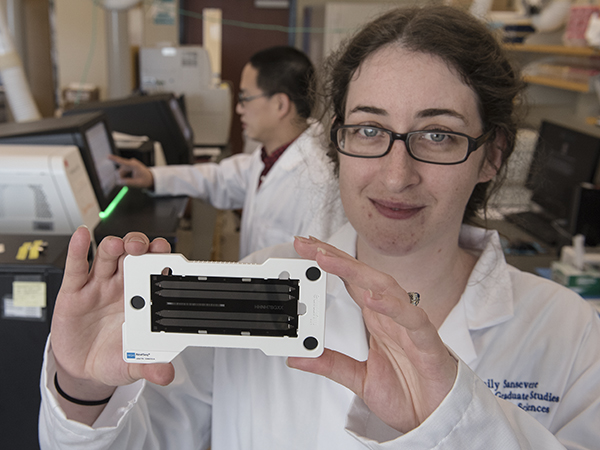
Emily Sansevere, a fifth-year graduate student, displays a genome sequencing chip. By loading millions of DNA fragments on a chip, she can then determine the entire genetic sequence, or genome, of a bacteria such as Staphylococcus aureus.
Since its discovery in the 1960s, Acinetobacter baumannii has become a “scary one,” said Dr. Larry McDaniel, professor and interim chair of microbiology and immunology.
“When it was first described in the Vietnam War era, it was pan-susceptible to antibiotics,” he said. “In less than 50 years, not only has it developed multi-drug resistance but also virulence.”
The species is a “critical” priority pathogen, the WHO list's top ranking. A study by McDaniel and others showed that A. baumannii strains can survive in blood, suggesting their ability to cause serious infections like sepsis.
Another “critical” priority is Pseudomonas aeruginosa. Or, as Dr. Richard O'Callaghan calls it, “Genghis Khan,” a merciless conqueror.
“The resistant strains are known to infect people with conditions like cancer, cystic fibrosis and severe burns,” said O'Callaghan, professor of microbiology and immunology. “These are people who don't need any more problems.”
The WHO's recent list aligns well with O'Callaghan's own top 10 “bad bugs” presentation that he has used in microbiology classes for years, including most of the species named in this story.
O'Callaghan's previous work aided in the creation of two antibiotics used for P. aeruginosa eye infections. He said new antibiotics are in the drug development pipeline for various species. However, it's been “decades” since the FDA has approved a new class, McDaniel said.
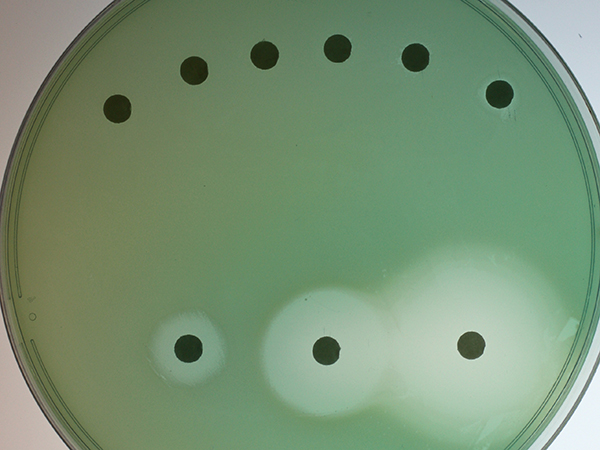
Pseudomonas aeruginosa colonies grow on a cell culture plate.
But while the WHO stresses the importance of new antibiotics, UMMC researchers said this is only part of the solution.
For example, Robinson cited a 2017 study that attributed a 30 percent decline in MRSA infections to better screening, hand washing and infection prevention emphasis at hospitals.
McDaniel said health care providers should also practice antimicrobial stewardship - “knowing when and how often to prescribe an antibiotic, and when to switch drugs if needed.” UMMC's own stewardship program helps providers make the optimal choices for their patients.
Everyone can take steps to prevent superbugs. The WHO recommends people follow their health care provider's instructions on antibiotic use and limit exposure to potential sources of infection.
By following these steps, we can all be heroes in the resistance against the superbugs.


Antimicrobial stewardship: “No one can do it alone”
Dr. Jason Parham, associate professor of medicine, leads UMMC's antimicrobial stewardship efforts. “Antibiotics are the only medication where use in one patient can impact the future effectiveness of the antibiotic in others,” he said. Watch the video below to learn about the problem of resistance and how UMMC clinicians are helping solve it.


