Office of Clinical Trials coordinates, optimizes research process
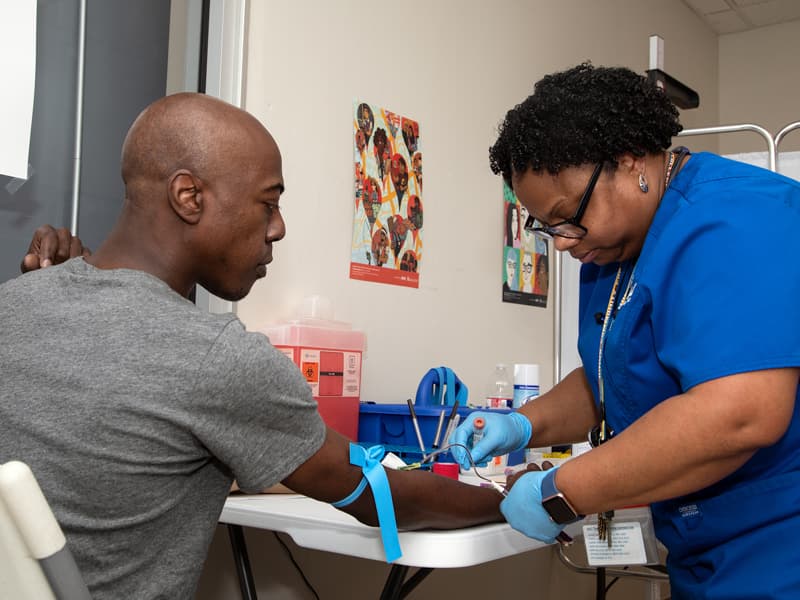
As the University of Mississippi Medical Center expands its research enterprise, a new office aims to make conducting clinical trials easier and more accessible to potential participants.
Formed this year, the UMMC Office of Clinical Trials serves to streamline the behind the scenes workings of this critical stage of the medical advancement process.

Whitney Bondurant, director of the Office of Clinical Trials, said that the group’s goals include making sure that research agreements are followed, standardizing training for clinical research staff, recruitment and data management, and making the set-up process more efficient.
“One of the purposes of this office is to take the administrative burden off study coordinators and administrators by using dedicated project managers,” Bondurant said. The OCT’s staff includes several veterans of other UMMC research-oriented offices, as well as new employees.
The idea for the office came from Dr. Richard Summers, associate vice chancellor for research, who wanted to provide additional resources for investigators.

Summers said that because UMMC’s status as an academic medical center that treats patients with a range of health conditions, “we have many opportunities to perform clinical trials that are supported by grants or industry. However, busy clinicians sometimes find that navigating the process for initiating these trials is difficult,” Summers said. “The Office of Clinical Trials is designed to provide support for those wanting to perform clinical trials at UMMC.”
The OCT’s first efforts center on making it easier to identify potential research participants and make the public more aware of research opportunities.
Introduced in June, the “Find a Clinical Trial” webpage includes information about ongoing studies at UMMC. The tool contains information on experimental studies of new drugs and procedures as well as observational studies that are recruiting participants. By clicking a trial name, users can view detailed information about the purpose of the study; participant inclusion and exclusion criteria; and contact information for the research team.
Bondurant said the interface improves on earlier efforts to bring visibility to ongoing clinical trials.
“People [in the general public] are often unaware of how they can learn more about enrollment or participation in clinical trials. We wanted to make the process more transparent, engaging and user-friendly,” she said.
The site’s design was inspired by clinicaltrials.gov, the National Institutes of Health’s database of clinical studies underway around the world. Users can filter through the list to find trials related to particular health conditions, recruitment criteria, study phase and locations where the study visits will occur.
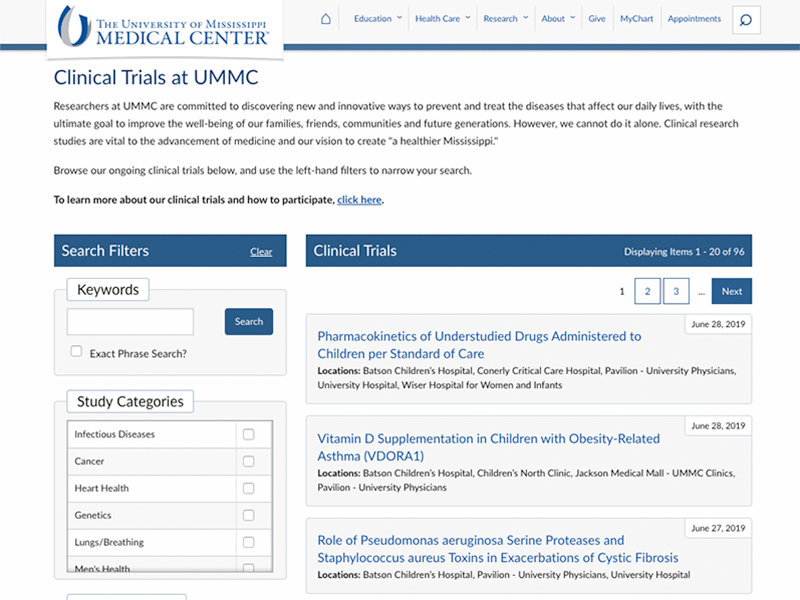
As of this writing, UMMC’s page lists 101 trials enrolling participants. They include multiple studies associated with cancers, cardiovascular diseases, neurological conditions and other diseases in both children and adults. The OCT will update the page regularly as new studies commence recruitment following Institutional Review Board approval.
People also can submit a general interest form stating their interest in receiving more information about future trials. This builds a pool of potential participants for future studies. People who are not current UMMC patients can request information and participate in studies as well.
On August 5, the OCT introduced a research participant recruitment application. Still in beta testing, it contains information about patients who give permission to be contacted about a research study. UMMC investigators who want to use the tool for IRB-approved research studies can fill out a request form. There will be an information session on the app on Friday, August 16 in the third floor conference room in the Translational Research Center.
Currently the OCT is using the principles of TACT, or transforming the activation of clinical trials, a Mayo Clinic initiative to increase the efficiency of clinical trial start-up. Through TACT, Mayo decreased the time it takes to process the agreement for an industry-sponsored clinical trial from 189 days to 65 days, a target UMMC also plans to reach.
Over the next year, the Office of Clinical Trials will roll additional initiatives. They are preparing a grant-funded standardized study coordinator training in collaboration with Pennsylvania State University and the Mayo Clinic, set to begin September 16-20. UMMC has also purchased Velos, a clinical trials management system, in conjunction with the Mayo Clinic. The shared system, which gives researchers the ability to track recruitment, milestones, billing and project budgets all in one place, will make it easier for the institutions to conduct research studies together.
“Velos will enhance what we already have been doing,” Bondurant said.
The Medical Center will start pilot tests of Velos later this year, with a campuswide rollout planned for summer 2020.
The Office of Clinical Trials is part of a broader movement within UMMC to build the logistical and physical infrastructure to advance clinical research. Later this month, the Medical Center will dedicate the Clinical Research and Trials Unit, a 22-bed inpatient/outpatient space on the seventh floor of University Hospital.
Together, these projects enhance clinical research and trials at UMMC, which in turn helps build a healthier Mississippi.
“One of the biggest benefits people report from participating in clinical research is a greater personal awareness of their health,” Bondurant said. “This can have a broader public health impact than access to experimental treatments alone."


