UMMC researcher: CT texture may improve melanoma survival predictions

Published in News Stories on September 21, 2015
Dr. Andrew Smith foresees a day when doctors can look at images of some metastatic melanoma tumors and better show whether that patient is benefiting from a particular treatment or not.
Smith and his colleagues saw their paper outlining the process published by the American Journal of Roentgenology in September, just a few weeks after former President Jimmy Carter announced that he is being treated for metastatic melanoma, or skin cancer that has spread to other places in his body.
Smith, a researcher and associate professor of radiology at the University of Mississippi Medical Center, said computed tomography (CT) scans can help show if angiogenesis inhibitors, drugs that can destroy blood vessel growth to tumors, are working. The drugs used in his study are not the same ones Carter is receiving.
He hopes the findings will add another tool to a limited toolbox for metastatic melanoma. One day the scans may give doctors the ability to predict metastatic disease response after only one cycle of therapy. If the tumor shows a favorable response, this is encouraging to patients and their doctors. If the tumor shows an unfavorable response, doctors and patients may switch to a different therapy. Long term, that could save the patient the cost of the drug, side effects from it and put them in a therapy that helps slow or destroy their cancer.
Smith said research doctors may also use this information to plan clinical trials and use the tool to bring certain drugs to market faster and at less cost.
"As we enter the age of personalized medicine, the ability and need to assess a cancer patient's individual response to his treatment becomes critical," said Dr. Timothy McCowan, professor and head of the Radiology Department. "Dr. Smith's research is on the forefront of providing this information."

Sheehan
Dr. Natale Sheehan, a medical oncologist in University Cancer Care's melanoma clinic, said she looks forward to any new tools to help treat melanoma. "With several new melanoma therapies on the horizon, having a tool that could predict response would definitely benefit patients and allow oncologists to tailor the treatment for each patient," she said.
"This is why multidisciplinary approaches to treating cancer are so important," said Dr. Srinivasan Vijayakumar, UMMC Cancer Institute director. "Our teams already rely on radiologists to tell us if tumors are growing or shrinking, but this adds a new dimension, literally, to how imaging can aid in treatment decisions."
Traditionally, radiologists, doctors who review scans from X-rays, CT, CAT, MRI and other imaging methods, can study images taken before and after therapy and tell if a tumor size is shrinking. Smith said in patients treated with angiogenesis therapy the tumor may not shrink but its appearance may change.
He's looking at the texture of the tumor instead of just its length and width to help determine whether treatment is working. The findings benefit patients in two ways:
- If therapy is working they and their doctors know to continue using it.
- If therapy isn't working, they and their doctors know more quickly to switch to something else and they avoid the side effects of anti-angiogenesis drugs.

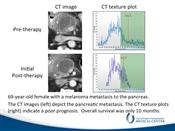
CT scans and histogram plots of a 69-year-old female with a melanoma metastasis to the pancreas. The CT images on the left show minimal change in the tumor after therapy begins but there is a striking change in the CT texture plots on the right that indicate a poor prognosis.
So what's he looking for?
"You can think of it like sandpaper," Smith said. "You can have fine sandpaper made of small granules or coarse sandpaper made of a mix of small and large granules. The fine sandpaper feels smoother because all of the granules are evenly spread out; the fine sandpaper is homogeneous. The coarse sandpaper feels bumpy because the granules are different in size or clumped together; the coarse sandpaper is heterogeneous. Tumor may change in size with some therapies but can change from homogenous (fine) to heterogeneous (coarse), or vice versa during treatment. CT texture allows us to measure this."
His research started with renal cell carcinoma, a kidney cancer. "We had success with metastatic kidney cancer and wanted to see if this would extend that success to other tumors. Melanoma is highly vascular, just like kidney cancer. I needed to find someone treating it with anti-angiogenic therapy. Ohio State had completed such a clinical trial. We got permission to review the images and patient data from those who participated in the study."
That allowed him to compare texture on the CT images taken before therapy with ones taken soon after it began and then compare what he saw with a patient's actual progress. The findings correlated. When he found particular textural changes in the imaging, the therapy was working. When he didn't see them (when the sand all looked alike) patients were not responding. This was particularly useful in patients who didn't have much of a change in tumor size after starting therapy.
The next task is to standardize how to read the images, Smith said.
Other researchers have spotted textural changes too, but each group of researchers seems to find different ways to measure texture (smoothness or coarseness). Smith and his team from the Society of Abdominal Radiology have found a new approach to this problem and are working to find the type of texture measures that are most reproducible. He plans to have multiple readers review images from multiple clinical trials conducted at different centers and incorporate a variety of tumors and treatments. Then his team and others can focus on the type of texture measures that are most suitable for incorporation into clinical trials.
"We're trying to standardize our approach to CT texture analysis," he said. Soon he and his team will submit a NIH grant to conduct this standardization on a larger scale.
His hope is if they can standardize the process it can be applied to multiple cancers treated with anti-angiogenesis drugs. Those might include particular types of colon, head and neck cancers, breast, renal cell GIST, lung and pancreatic neuroendocrine cancers.
Soon he expects to receive a 3D imaging phantom (a life-like fake body) with fake tumors. After running some tests with it, he hopes to further refine the best CT texture analysis methods. He will do this by scanning the 3D imaging phantom on 20 or more CT scanners from eight different institutions. He wants to make sure that the CT texture parameters his team and others will use in clinical trials are optimized to any type of CT scanner from any manufacturer.
If radiologists can provide consistent results using the guidelines Smith and his research group provide, the results will be soon available in clinical trials. "We have to have reproducible results," he said. "It has to be as reproducible as measuring the length of the tumor."
The process could take five to seven years to do the testing, gather the information and publish the results. It could be another three to four years before the idea is usable in clinics, he said.
First it will have to be proven effective at predicting response and the final step would be receiving FDA approval. He's trying to make sure he's covered some of those FDA bases as the research continues.
The paper, "Predicting Overall Survival in Patients With Metastatic Melanoma on Antiangiogenic Therapy and RECIST Stable Disease on Initial Posttherapy Images Using CT Texture Analysis," was published in the September issue of the American Journal of Roentgenology. Others working on this paper include Mark R. Gray, Darya Shlapak and Xu Zhang, UMMC: Sara Martin del Campo and William E. Carson III, Department of Surgery, The Ohio State University Comprehensive Cancer Center in Columbus, Ohio; and Balaji Ganeshan, Department of Biomedical Engineering, University College of London, London, UK.