Study Activation
Main Content
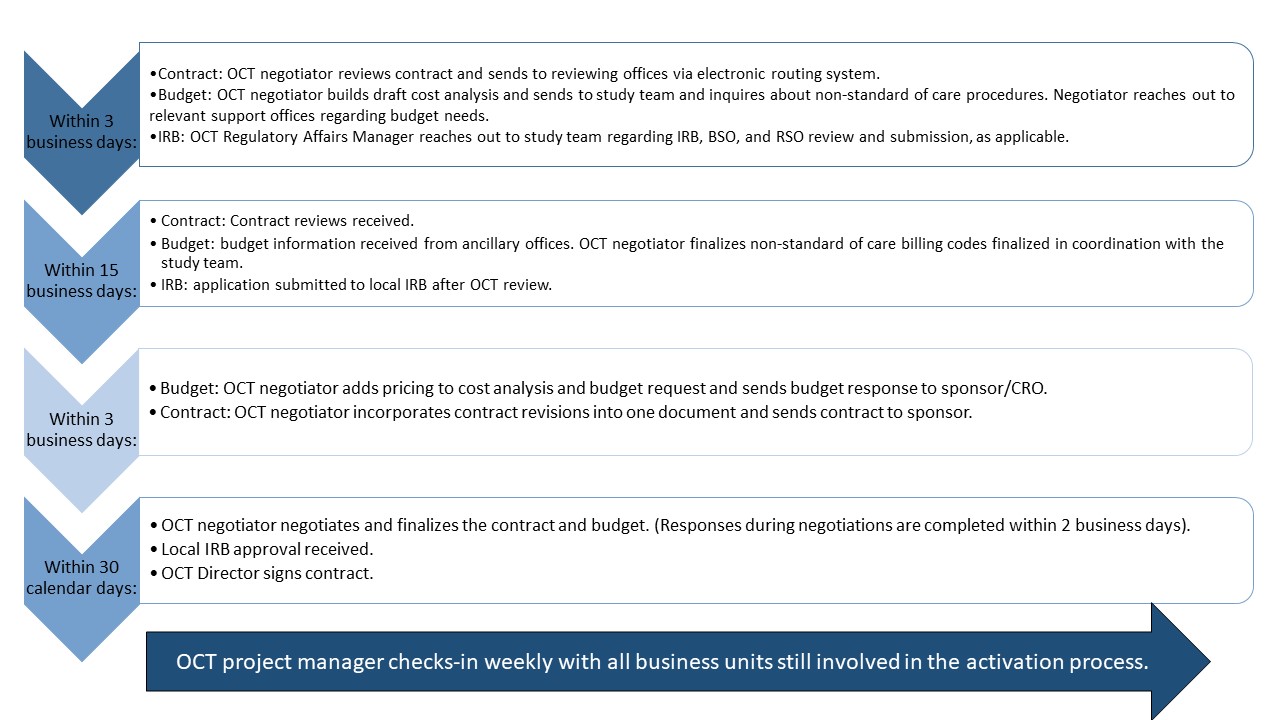
Activation of Clinical Trials (ACT) Process
Definition
- The ACT process governs the activation of clinical research studies and includes contract, budget, and IRB approval. This streamlined, 65-day process seeks to eliminate silos, redundancies, and non-value-added time.
Policies/guidelines
- The ACT process starts once all relevant documents are received. The Study Information Sheet (SIS) outlines these documents. Ensure that the documents are completed and received by the Office of Clinical Trials (OCT) in a timely manner so as not to delay the activation process.
- The graphic below illustrates the various components of the ACT process and relevant timelines.