
UMMC provides patient-centered treatment, clinical excellence, and an advanced level of care unavailable anywhere else in the state.

for Kids.
Children’s of Mississippi is here for every child
with the state’s only children’s hospital
plus clinics statewide.
Latest News

70 Years of Pharmacology: Past, Present, Future
Tuesday, January 20, 2026
As the Department of Pharmacology and Toxicology marks its 70th anniversary, it stands as a powerhouse with a legacy shaped by decades of discovery, leadership and transformation. Read More

Porter Antici named 2026 Children’s Miracle Network champion for Mississippi
Tuesday, January 20, 2026
Children’s of Mississippi announced Friday that Porter Antici has been named Mississippi’s 2026 Children’s Miracle Network Champion, a role in which the 6-year-old will serve as an ambassador for children receiving care across the state. Read More
Latest Articles

See, Test and Treat offers free cancer screenings for uninsured, underinsured
Published on Monday, February 9, 2026

C Spire donates $5M for new UMMC cancer center
Published on Monday, February 9, 2026

That’s My Job: Orthopaedic Researchers
Published on Monday, February 9, 2026
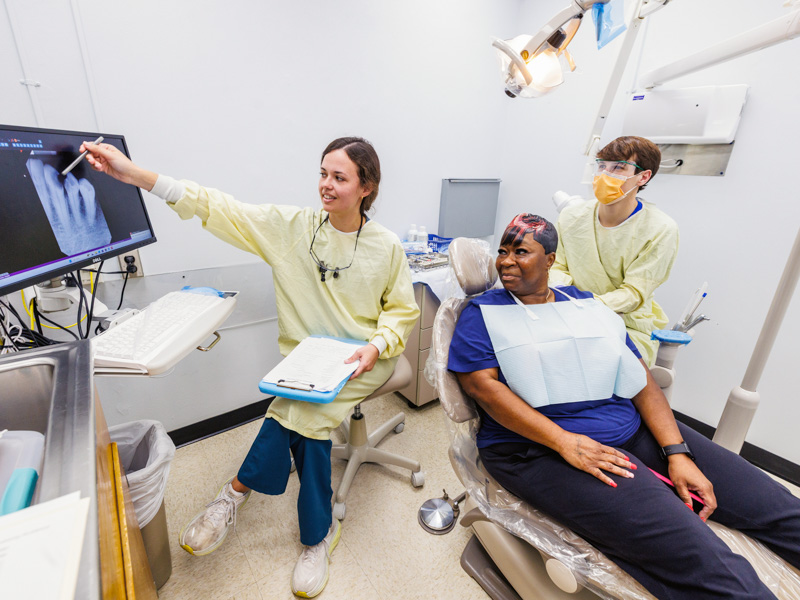
Dental Mission Week celebrates a decade of expanding access to care
Published on Monday, February 9, 2026

Solutions to violence in Mississippi evident in UMMC research efforts
Published on Monday, February 9, 2026

Residencies in PT expand in sports, orthopaedics
Published on Monday, February 2, 2026

Parkers’ $250,000 gift advancing cancer care at UMMC
Published on Monday, February 2, 2026

Children’s of Mississippi ambassador designs 2026 Run the Rainbow medal
Published on Monday, February 2, 2026
Find Us on Social Media
Get news and information you need about the Medical Center by connecting with us through our social media community. You’ll find events, news stories and campus activities shared daily. UMMC is active on Twitter, Facebook, Instagram, YouTube and LinkedIn.
You may also connect with the Children’s of Mississippi social media community on Twitter, Facebook, Instagram and YouTube.
Follow Dr. LouAnn Woodward on Twitter.
We look forward to hearing from you!
Sign Up for Consult

Connect with UMMC via new e-magazine
In CONSULT, readers will have the opportunity to learn more about the cutting-edge clinical advances, innovative educational programs and groundbreaking research occurring at UMMC. Plus, CONSULT will regularly showcase our experts' best advice for living a healthy and mindful life.






