- Administration
- Outreach Programs
- Mississippi Center for Emergency Services
- Research
Research
CriSP-HS: The Cold Stored Platelet Early Intervention for Hemorrhagic Shock Trial
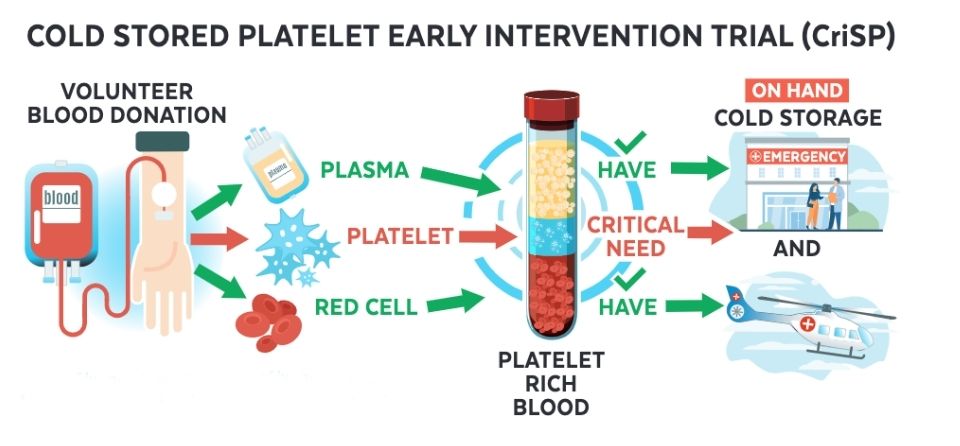
From volunteer blood donors to cold-stored platelets
Blood platelets are collected from volunteer donors, processed into plasma, platelets, and red cells, then cold-stored in ERs for quick access for critically injured patients..
A LITES Network Study
A research study comparing cold-stored platelets to usual transfusion in injured patients with major bleeding
What is CriSP-HS?
The Cold-Stored Platelet Early Intervention in Hemorrhagic Shock study, or CriSP-HS, will study whether giving cold-stored platelets early to patients who are losing a lot of blood will help stop the bleeding better than standard care.
For this study, some people who have serious injuries and who are losing a lot of blood will get cold-stored platelets. Others will get traditional care, which could include room temperature platelets. Researchers will look at both groups to see if one group did better and will collect information from those patients’ health records as part of the study. Researchers believe the risk to patients are the same whether getting cold stored platelets or room temperature platelets, but all the facts are unknown.
Benefits and Risk
Researchers think cold platelets may work better than room temperature platelets when trying to stop serious bleeding. Giving cold platelets sooner during emergency treatment may also help get bleeding under control faster.
Risks are rare and include infection, allergic reaction, fever, and shortness of breath. Researchers do know that cold-stored platelets are less likely to have infection-causing bacteria than room temperature platelets. It is not known if getting cold-stored platelets increases the risk for too much clotting, or whether the cold storage makes other risks better or worse.
Why Cold-Stored?
Soon after collection, the laboratory cools down blood platelets and ‘cold-stores’ them until they are given to a patient. Because cold-stored platelets do not need to be brought to room temperature, they can be given to patients earlier during the emergency treatment.
New studies show that cold-stored platelets may work better to stop serious bleeding than room temperature platelets. And giving cold platelets to patients sooner might also be helpful.
Researchers believe the benefits of the CriSP-HS study may help improve the survival rate for emergency patients who have serious injuries.
Linking Investigations in Trauma and Emergency Services (LITES)
LITES is a network of US trauma systems, medical professionals, prehospital providers and emergency medical services who join together doing research on emergency injury-care and outcomes. The University of Mississippi Medical Center is participating in this study because of its experience in emergency trauma, helicopter critical care transport and blood product transfusion protocols and practices.
What is EFIC?
Research studies like this use a process called Exception from Informed Consent (EFIC).
In studies about emergency treatment, patients are usually too sick or injured to give us permission to include them in the study before emergency treatment begins.The most important task is always to stop the bleeding and try to save the patient’s life. We do tell the patient, or a family member, about the study as soon as we can and ask their permission to keep them in the study.
Emergency treatment studies like this one must follow special rules and be carefully reviewed by several groups to ensure no one is taking advantage of very sick patients.
Study leaders
Principal Investigator
Jason L. Sperry, MD, MPH
University of Pittsburgh Medical Center
Linking Investigations in Trauma and Emergency Services (LITES) network
Site Investigator
Matthew E. Kutcher, MD, MS
UMMC Department of Surgery
mkutcher@umc.edu
415-846-0856
Project Manager
Meg Meagher
smeagher@umc.edu
601-815-5533
Study Sponsor
Department of Defense
- Expected Study Enrollment: 200
- Expected Site Enrollment: 40
Complete the CriSP-HS Survey
To complete a survey about the CriSP-HS project, click here.
For questions or to learn more about this study:
- Visit: www.litesnetwork.org/CriSP-HS
- Email: CriSP@edc.pitt.edu
- Call: (800) 664-0557


