Coronavirus, COVID-19 Information
The University of Mississippi Medical Center responded to the COVID-19 pandemic by taking aggressive steps to increase testing, promote vaccination, offer the best available treatments and conduct research that serve patients and families now and in the years to come.
COVID-19 News
COVID-19 News Feed
Batson Kids Clinic now offers COVID-19 vaccinations for children 6 months and older
Monday, Jul. 11
RECOVER explores Long COVID
Monday, Feb. 28

COVID-19 vaccination during pregnancy protects babies, research finds
Thursday, Feb. 17
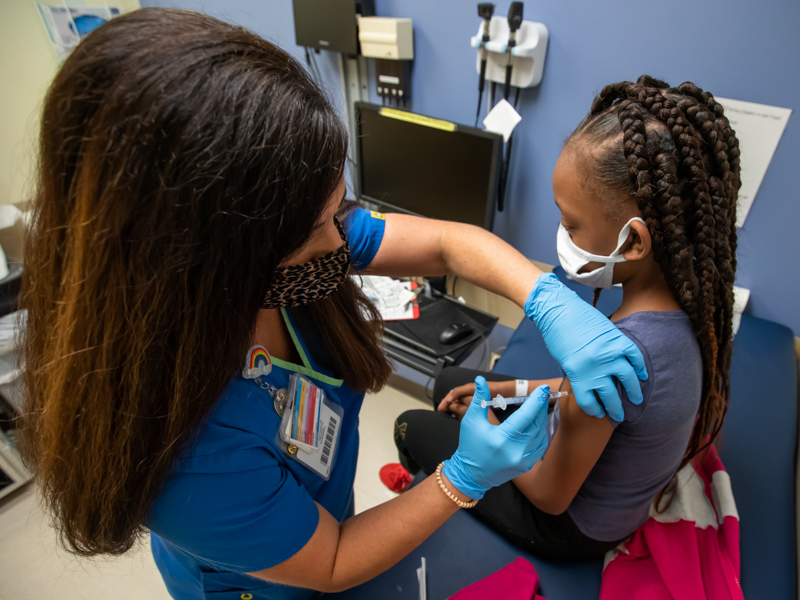
Unvaccinated kids bear brunt of COVID-19, studies show
Monday, Jan. 24
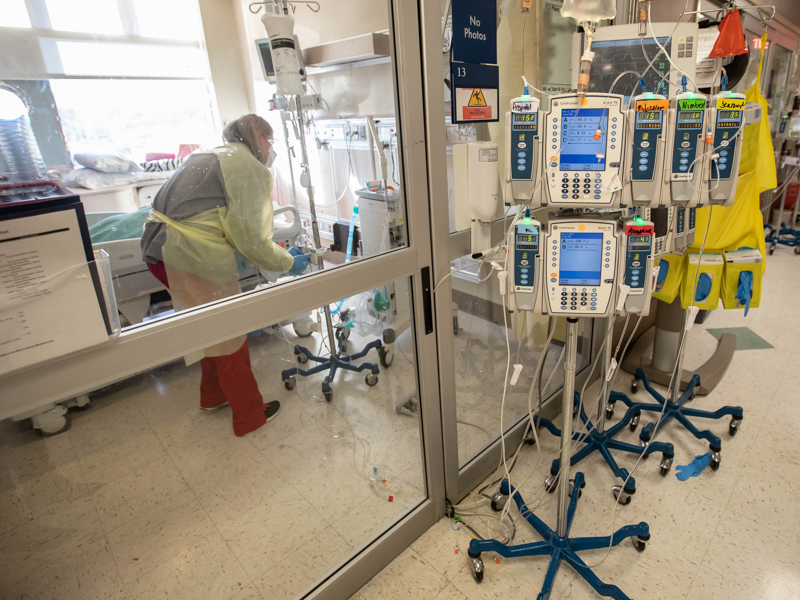
From COVID-19 variant to variant, critical care providers fight to save lives
Monday, Jan. 24
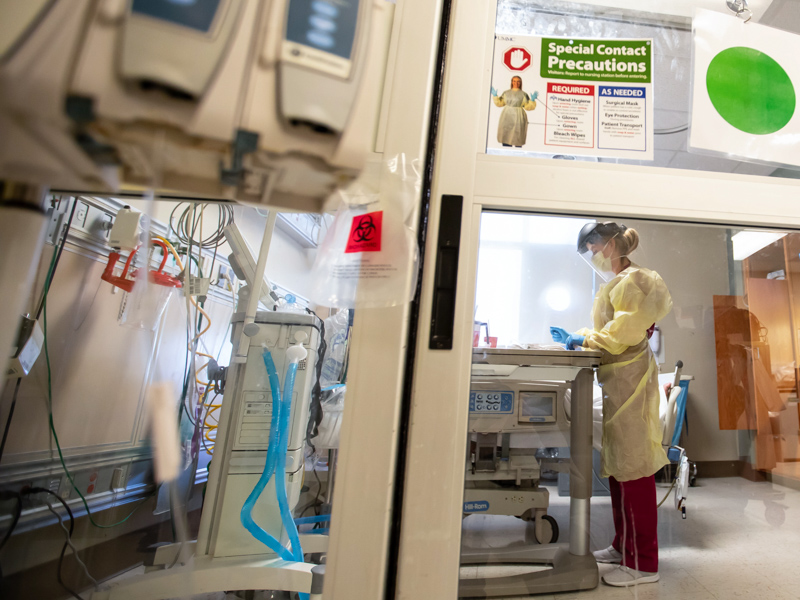
Omicron surge creates unprecedented staff shortages as hospitalizations rise
Tuesday, Jan. 11

Omicron and Delta variants: What do we know?
Monday, Jan. 10
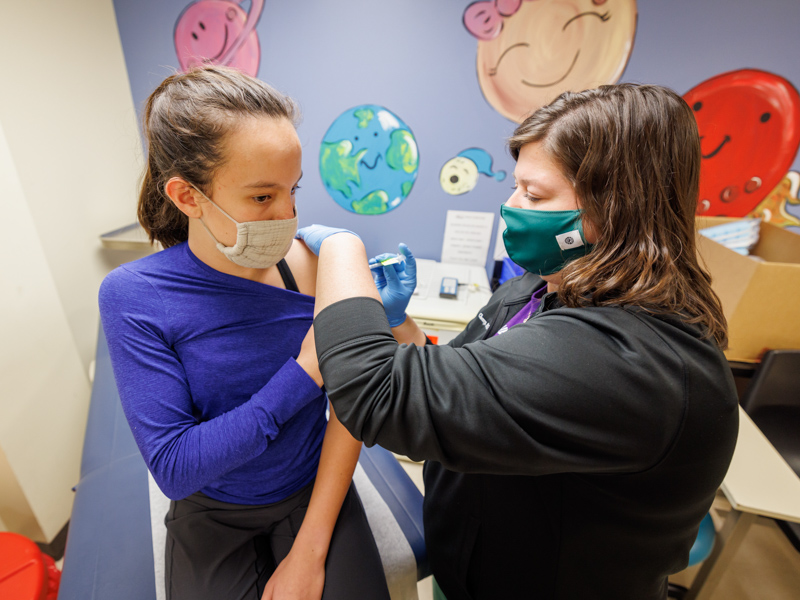
Majority of children hospitalized with COVID-19 are unvaccinated
Monday, Jan. 10
Show More News
Resources