


Cancer Center and Research Institute
The Cancer Center and Research Institute unites physicians, nurses and researchers from the University of Mississippi Medical Center and the University of Mississippi in the quest to stop cancer through:
- Interdisciplinary world class cancer prevention, detection and treatment.
- Research to better prevent, detect and treat cancer.
- Education for Mississippi residents and the medical caregivers who one day will provide their cancer care.
Adult and pediatric physicians see more than 2,000 new patients annually and follow more than 5,800 survivors. Cancer teams see many people with rare or complex care needs.
Featured News
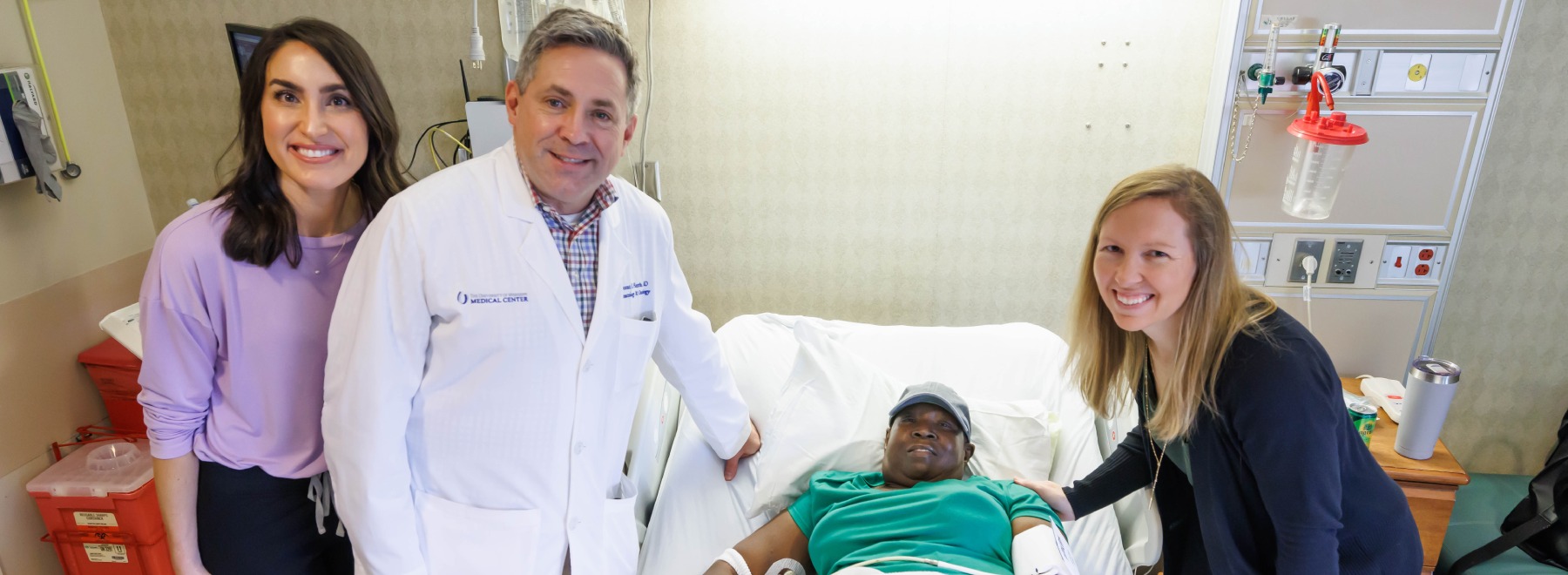
Cancer journey extends UMMC professor’s lessons beyond classroom
Monday, December 29, 2025
For decades, Dr. Stanley Smith has shaped generations of future physicians at UMMC. When he became a cancer patient, that lifelong commitment came full circle, deepening his trust in the care he has long championed. Read More
![The ACT Center team poses for a photo, from left, [front row] Dr. Jonathan Hontzas, director; Sharon Terry, tobacco treatment specialist; Kelli Grant, manager of research operations; Brittany Tichenor, tobacco treatment specialist; Erica Cameron, lung cancer screening coordinator; Latoya Scott, scheduler; Stacy Wilson, medical office assistant; Leslie Musshafen, associate director of administration for the Cancer Center & Research Institute; [back row] Langston Moore, project manager for the Cancer Center & Research Institute; Kathy Banks, administrative assistant; and Jay Thompson, project manager.](/news/News_Articles/2025/12/images/ACT-Pinning-20251212-22a.jpg)
ACT Center holds pinning ceremony honoring tobacco treatment graduates
Monday, December 15, 2025
Last week, the ACT Center for Tobacco Treatment, Education and Research celebrated over 40 individuals who have committed to a tobacco-free lifestyle during a pinning ceremony for the program’s graduates. Read More
Latest News

CCRI research seeking to unlock mysteries of pancreatic cancer with $1.8M NCI grant
Published on Monday, December 8, 2025

Cancer care questions met with answers from UMMC experts
Published on Monday, November 3, 2025

Front and Center: Dr. Devika Das
Published on Monday, November 3, 2025

‘Too much more to offer’: CCRI breast cancer patient’s journey underscores early detection
Published on Monday, October 13, 2025

Delta cancer rates move McRights to make significant gift to CCRI campaign at UMMC
Published on Monday, October 6, 2025

Hardin Foundation pledges $2.5M for “once-in-a-generation” UMMC cancer center expansion
Published on Tuesday, September 2, 2025

UMMC’s See, Test and Treat promotes preventative health care with free cancer screenings
Published on Monday, March 31, 2025
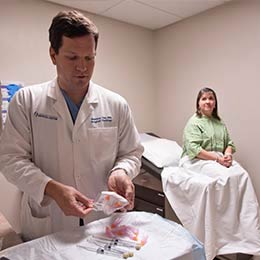
Treatment
Interdisciplinary teams meet regularly to review each new patient’s case and recommend the best treatment for each individual. Doctors here see many unusual and complex cases.
Health Care
Education
The Cancer Center and Research Institute offers the state’s only residency and fellowship programs for professionals seeking a career in cancer care.
Students and Trainees
Research
Researchers and physicians at UMMC and at the University of Mississippi are working together to seek new and better ways to prevent, detect and treat cancer.
Research Overview

